-
PDF
- Split View
-
Views
-
Cite
Cite
Roger A. Powell, Gilbert Proulx, Trapping and Marking Terrestrial Mammals for Research: Integrating Ethics, Performance Criteria, Techniques, and Common Sense, ILAR Journal, Volume 44, Issue 4, 2003, Pages 259–276, https://doi.org/10.1093/ilar.44.4.259
Close - Share Icon Share
Abstract
We propose that researchers integrate ethics, performance criteria, techniques, and common sense when developing research trapping programs and in which members of institutional animal care and use committees address these topics when evaluating research protocols. To ask questions about ethics is in the best tradition of science, and researchers must be familiar with codes of ethics and guidelines for research published by professional societies. Researchers should always work to improve research methods and to decrease the effects on research animals, if for no other reason than to minimize the chances that the methods influence the animals' behavior in ways that affect research results. Traps used in research should meet performance criteria that address state-of-the-art trapping technology and that optimize animal welfare conditions within the context of the research. The proposal includes the following criteria for traps used in research: As Criterion I, killing-traps should render ≥ 70% of animals caught irreversibly unconscious in ≤ 3 min (calculated with 95% confidence). As Criterion II, live-traps should trap ≥ 70% of animals with ≤ 50 points scored for physical injury (calculated with 95% confidence). The types of traps described include killing-traps (snap traps, rotating jaw traps, snares, pitfalls, and drowning sets), common sets, and the common types of live-traps (box and cage traps, pitfalls, foothold traps. snares, corrals and nets, and dart collars). Also described are trapping methods for specific mammals, according to which traps fulfill Criteria I and II for which species, and techniques for short-term, long-term, and permanent marking of mammals.
Guiding Concepts
Good research on free-living wild animals increases our knowledge of animals and helps wildlife professionals to develop effective conservation and management programs. To be consistent with these ends, research must use sound design to test hypotheses and to answer specific questions while making certain that the research does not have negative effects on the animals that influence research results. Much research on wild mammals requires trapping and marking of animals. We present here recommendations for trapping and marking mammals in research designed to gain scientifically sound information and designed to minimize unwanted, negative impacts on the individual mammals and populations being studied.
Our approach integrates ethics (professional values and conduct), performance criteria, techniques (traps, marks, and methods) and common sense (sound practical judgment). We propose that researchers address these topics when developing trapping programs and when submitting research plans to institutional animal care and use committees (IACUCs 1 ). We also propose that IACUCs address these topics when evaluating research protocols. We identify performance criteria for traps, with the caveat that when no available trap meets the recommended criteria and the research is sound, researchers should use traps that best meet research and ethical concerns. The major types of traps and sets for the capture of mammals are discussed below. We present first an overview of traps, which is best done by grouping similar traps. We then present the best methods of trapping different mammals, which is best done by grouping similar species. This approach results in some redundancy but minimizes confusion because some mammals can be trapped using very different traps, similar traps can be used to trap very different mammals, and traps that meet performance criteria for one species may not for other. In the end, our two lists are, perforce, incomplete because responsible researchers strive continuously to improve traps to work more efficiently, more selectively, and more safely for both animals and people.
Ethics
Many philosophers have argued that ethics must be an essential component of how humans treat animals (e.g., Regan 1983 ; Singer 1975 ). Since the early 1990s, many biologists have argued forcefully that treatment of animals in research must meet ethical standards and that field biologists must address some sticky questions (e.g., ASM/ACUC 1998 ; Bekoff 2000a , b , 2001 , 2002 ; Bekoff and Jamieson 1996 ; Berger 1998 ; Berger et al. 2001 ; Cuthill 1991 ; Elwood 1991 ). Bekoff (2002) noted that to ask questions about ethics is in the best tradition of science. Researchers must always work to improve research methods and to decrease the effects on research animals if for no other reason than to minimize the chances that research methods affect the animals' behavior in ways that ultimately influence research results.
All researchers should be familiar with the codes of ethics and the guidelines for research published by professional societies devoted to research on animals. The American Society of Mammalogists ( ASM/ACUC 1998 ) and the Association for the Study of Animal Behaviour and the Animal Behavior Society ( ASAB/ABS 2000 ), for example, outline research methods that these societies find ethically appropriate. These society guidelines are updated appropriately and are intended not to obstruct research but instead, to establish a context for the continued quest to improve research design ( ASM/ACUC 1998 ). All researchers should also be familiar with laws and national standards that affect their research, such as (but not limited to) the Animal Welfare Act and the Endangered Species Act in the United States, The Animals (Scientific Procedures) Act in the United Kingdom, and the Guide to Care and Use of Experimental Animals in Canada ( CCAC 1984 , 1993 ). Some societies (e.g., ASAB/ABS 2000 ) base decisions to publish submitted manuscripts in part on whether the research is consistent with the societies' codes of ethics.
Research design should minimize potential long-term effects of trapping ( Seddon et al. 1999 ) and deal with nonrandom sampling ( Banci and Proulx 1999 ). For example, because adult males, dominant individuals, and juveniles are usually trapped first, research using killing-traps can affect population structure of the remaining animals in a nonrandom way. Bekoff argued that researchers should approach research with the basic principles used in everyday life: "Do no intentional harm, respect all life, treat all individuals with compassion, and step lightly into the lives of other beings, bodies of water, air, and landscapes" ( Bekoff 2002 , p. 23). He challenged researchers to consider such questions as "What happens in both locations when individuals are moved from one place to another?" When animals are moved, what is the effect on the remaining members of their species, what are the effects on the social organization of that species, and what are the effects on the integrity of the community? Research should be designed, as practicable and without affecting research goals, to minimize effects on all levels from the individual animals trapped, through social groups and populations, and to communities. Researchers have repeatedly noted that if trapping must continue to be a tool for research and wildlife management, trap technology and management programs must evolve with public sentiments and conservation objectives ( Proulx and Barrett 1994 ; Schmidt and Bruner 1981 ).
Absolutely minimizing the effects of research and trapping on animals may conflict with research logistics or may cause research costs to exceed available funding. No simple guidelines exist regarding how to weigh the effects of research (positive and negative) on individuals versus groups versus populations versus communities. Likewise, no guidelines exist about how to weigh research funding versus effects on animals (injury) versus importance of results (e.g., for individual animals, animal populations, and humans; however, see also the discussion by Bekoff and Jamieson 1996 ). Most often, research results have long-term positive effects on populations, and hence to individuals in the future, whereas the individual animals studied and their social groups receive the brunt of negative effects.
Negative effects on animal subjects of field research must, however, be considered in context. Humans are now all pervading. To assume that humans will have no effects on animals if research is not done (thus eliminating negative effects on animal subjects of field research) is just as unethical as ignoring the negative effects of research on animals when research is done. Researchers must be able to argue convincingly that the potential positive effects of their research exceed the potential negative effects.
Criteria for Trap Performance
How animals are trapped and the impact of trapping on mammal populations are major societal concerns ( Proulx and Barrett 1989 ). Traps used in research should meet performance criteria that address state-of-the-art trapping technology and that optimize animal welfare conditions within the context of the research. In most cases, minimal impact of trapping on research subjects leads to minimal aberrant impact of trapping on research results.
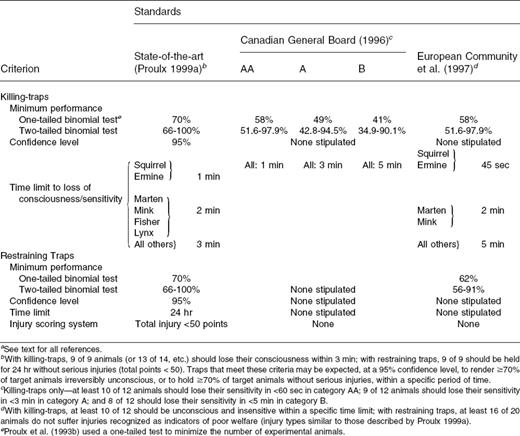
Setting performance criteria for killing-traps is arguably easier than setting performance criteria for restraining traps because unconsciousness and death are relatively easy to define objectively, compared with the injury, anxiety, and hardship that may be experienced by restrained animals. The Canadian General Standard Board ( CGSB 1984 ) adopted the criterion that a humane killing trap must render an animal unconscious and unable to recover within 3 min. To some ethicists, 3 min is unduly long, yet it is a realistic time that pushes current technology. When it can be reduced, it will be. Proulx and Barrett (1994) proposed the following criterion for adoption:
Criterion I - for Killing-Traps: State-of-the-art killing-traps should, with 95% confidence, render ≥ 70% of animals caught irreversibly unconscious in ≤ 3 min.
Despite solid technical advances in trap research and development that meet this criterion ( Proulx 1999a ), recently developed standards ( CGSB 1996 ; ECGCGRF 1997 ) have not completely incorporated those technical advances ( Table 1 ). The United States has not signed a binding international agreement to adhere to standards of humane trapping; it will, instead, develop its own best management practices on the basis of technical, economical, and social criteria ( IAFWA 1997 ).
Comparison of trapping standards based on successful state-of-the-art trap research and development ( Proulx 1999a ) with those of the Canadian General Standards Board (1996) and the European Community et al. (1997)a
 |
 |
Comparison of trapping standards based on successful state-of-the-art trap research and development ( Proulx 1999a ) with those of the Canadian General Standards Board (1996) and the European Community et al. (1997)a
 |
 |
Proulx and Barrett's (1994) criterion for performance of killing-traps has been used successfully in programs of trap research and development. To date it is the best defined, objective, and published criterion consistent with state-of-the art technological development. Some available traps meet this criterion for use with many mammals ( Proulx 1999a ).
Much, if not most, research on wild mammals that involves trapping uses live-, or restraining, traps. Tullar (1984) , Olsen et al. (1986) , and others (summarized by Proulx 1999a ) developed systems to score injuries caused by live-traps. Olsen and colleagues proposed scoring each bruise, minor cut, and joint damage between 5 and 50 points, depending on severity; scoring serious damage >50 points, increasing with increased severity; and scoring severe damage >125 points. They set a threshold at a total of 50 points. Mammals, however, respond to capture both behaviorally and physiologically ( Kreeger et al. 1990 ; Proulx et al. 1993a ; Seddon et al. 1999 ; Warburton et al. 1999 ). To date, no objective scoring system for live-traps integrates physical injuries with behavioral and physiological responses ( Proulx 1999a ), at least in part because interpreting such responses is not straightforward ( Dawkins 1998 ). For live-traps, we adopt a criterion parallel with that for killing-traps:
Criterion II - for Live-traps: State-of-the-art live-traps should, with 95% confidence, trap ≥ 70% of animals with < 50 points scored for physical injury.
When reasonable, researchers should collect data not only on physical injury but also on behavioral and physiological responses (e.g., Seddon et al. 1999 ; Warburton et al. 1999 ). In addition, they should be able to argue convincingly that their traps minimize both physical injury and negative behavioral and physiological responses compared with other traps that are also consistent with the research goals and logistics.
For both killing- and live-traps when no available trap meets these criteria, researchers should use traps that approach the criteria best. They also should minimize the number of animals captured if appropriate to research design, and should incorporate research to improve trap design within their research programs. For good research that is well designed and otherwise deserving of IACUC approval, a lack of traps that meet Criteria I or II must not be grounds, in and of itself, for an IACUC to fail to approve a protocol.
Procedures and Methods
Sample Size, or Number of Individuals That Must Be Trapped
Occasionally field research design does not require maximal numbers of animals to be trapped. When this is the case, researchers can often estimate a priori the numbers of animals that must be trapped and choose the research effort needed to test hypotheses, to meet management goals, or to reach objectives for animal control. For most mammal species, or for closely related species, the literature contains information on trapping success and summary statistics for sex ratios and age distributions of animals trapped. Researchers can estimate sample sizes needed for statistical significance and for adequate statistical power using information from the literature, from pilot data, or from both.
True experiments require random assignment of multiple (≥ 2) treatments (one of which might be a control) and replication ( Ratti and Garton 1994 ). Not all research requires experiments, but nearly all research requires appropriate replication. If research questions relate solely to individual animals, then the unit of measure that must be replicated is individuals. If research questions relate to social groups or to populations, then those units require replication. To replicate individuals in an attempt to answer population-level questions is pseudoreplication ( Hurlbert 1984 ), which can lead to conclusions that are critically misdirected and incorrect. When variation among individuals is limited, however, group and population questions can sometimes be answered with adequate replication of individuals ( Still 1982 ). In other words, variation among individuals can be used to estimate variation among groups or among populations when variation among individuals is low across all groups or populations. Replication of populations is often impossible for large mammals, necessitating the (cautious) use of individual variation to estimate population-level effects. Nonetheless, the validity of inferences from small samples is not straightforward ( Hurlbert 1984 , Kroodsma 1989 , McConway 1992 , Searcy 1989 ) and requires solid support.
Sample size on the correct level (individual, group, or population) can often be minimized in one of the following ways: by designing research that will yield data appropriate for statistical tests needing small samples (the tests may be parametric, nonparametric, or Bayesian); by using factorial design to explore the effects of several variables in one experiment; by using sequential and multivariate statistical methods; or by using repeated measures design ( McConway 1992 ). In the end, for most field research involving trapping, increasing sample size increases statistical rigor, which is urgently needed.
Handling
Handling of trapped individuals should minimize impact on the individuals. Quick handling often, but not always, minimizes impact. In general, but not universally, using anesthesia reduces the stress of being handled. Anesthesia may or may not, for example, reduce capture myopathy in ungulates ( Beringer et al. 1999 ; Kock et al. 1987 ; Read et al. 2000 ). Handling mammals without anesthesia sometimes returns animals to their social groups most quickly and allows quick release without danger of predation after handling. Yet, even for those species, such handling may cause abnormal behavior long after release and can reduce recapture success. When anesthesia is used and recovery is not rapid, trapped animals may need food, water, and other resources as well as protection from predation, weather, and other effects until they can be returned to the wild (e.g., Gehring and Swihart 2000 ; King 1973 , 1975 ).
Before using anesthesia, a researcher should gain experience with administration and monitoring and should consult with a wildlife veterinarian. When small mammals are handled, body and appendages should be restrained while allowing easy breathing ( ASM/ACUC 1998 ). Small mammals can be restrained in cloth, mesh, or heavy plastic bags, and the latter may be used to administer anesthesia if necessary.
Common Sense
Researchers must use common sense about trapping mammals, which implies that a researcher will judge appropriately the methods required for a study. Many research goals that required trapping even in the 1990s can now be achieved without trapping. When appropriate data can be collected more easily and inexpensively without trapping, common sense maintains that trapping should be avoided ( Bekoff and Jamieson 1996 ). Presence/absence data can be collected using track plates, remotely triggered cameras, and hair traps ( Zielinski and Kucera 1995 ). Remotely triggered cameras allow one to identify individual animals that received obvious markings when trapped a first time, which decreases the need for extended trapping. DNA obtained from tissue from hair follicles allows identification (albeit sometimes with unacceptable expense) of each individual that has left hair in hair traps ( Woods et al. 1999 ).
Mammals that can be observed readily can often be identified as individuals using natural markings ( Kelly 2001 ; Mukinya 1976 ; Pennycuick 1978 ). Outfitting large mammals with collars bearing remotely discharged, anesthetizing darts reduces necessary retrapping, reduces the capture of nontarget species and individuals, minimizes the probability that target animals will not be recaptured, and reduces the trauma and stress of recapture ( Powell et al. 1997 ; Powell, unpublished data). In addition, data and summary statistics in the literature can sometimes be used with new statistical and modeling techniques to test hypotheses without new field research. Replication of research is central to good science, and replication of important results should never be discouraged. Unnecessary duplication differs from replication, however, and should be avoided. Repeating research that has been replicated many times already, in the absence of significant, new permutations, is considered duplication.
The choice of live- (restraining) versus killing-traps depends, at the least, on research goals, research design, and study site. Both live-capture and kill-trapping can contribute to important research on evolution, ecology, animal behavior, physiology, parasitology, genetics, and other disciplines. Live-traps allow the live release of trapped animals, including nontarget animals. If, for example, pets or members of an endangered species have a reasonable probability of capture, then live-traps are dictated. When nontarget captures are unlikely, holding an animal in a live-trap only to kill it later to collect a sample may be less humane than using a quick-killing trap. Keeping animals alive may be required, however, to avoid freezing or decomposition of tissues to be sampled ( Kreeger et al. 1990 ).
Both live-traps and killing-traps can be monitored remotely; however, doing so may be unreasonably expensive, especially when traps can be checked easily in person multiple times daily. Remotely monitored traps must be visited regularly for maintenance because animals may avoid capture but still disturb trap sites and render the sets ineffective. Remote monitors must be set so that monitor failure causes traps to be checked. The device described by Graf et al. (1992) , for example, fails to meet this safety criterion because trapping an animal activates the monitor. If this monitor fails, animals can be trapped and the researcher not be notified.
Killing animals is important for some research on evolution and systematics, for wildlife management, and for animal damage control. Without the continuous collection of new specimens, museum collections cannot document changes in genetics, populations, species, and species' ranges. Mammal specimens should be deposited in museums that meet the standards set by the American Society of Mammalogists ( ASM/CSC 1978 ).
Whether using killing-traps or live-traps, common sense dictates choosing traps that maximize both selectivity and efficiency ( Pawlina and Proulx 1999 ). Selective traps minimize the capture of nontarget species or nontarget individuals within a species, thereby increasing the rate of data collection and reducing the overall impact of the research on the ecological community in the study area. Efficient traps have high capture rates and thereby lead to rapid data collection.
Finally, as practicable for the research, researchers should choose traps that minimize pain and discomfort. Mammals are sentient and have the ability to perceive injurious stimuli ( Kitchell and Johnson 1985 ). A trapping program should avoid discomfort as much as possible if for no other reason than not to do so can lead to negative affects on the animal subjects that ultimately affects the research results.
Traps
Traps used to capture mammals are diverse. They can be divided into several general types, yet tremendous variation exists within types. In this article, we present the major types; note, however, that Proulx (1999a) presented more detailed descriptions of some traps and described some test results. The list herein must be incomplete because responsible researchers are always striving to improve traps. Where traps have been evaluated for general performance, we report the results below. Where performance is specific to individual species, we report the results with information about trapping individual species.
Live-Traps
Capture devices classified as live-traps include box and cage traps; pitfall traps; foot-hold traps; foot-, neck-, and body-snares; and corrals and nets. A brief description of each device appears below.
Box and Cage Traps
Box traps restrain animals inside a box with solid wood or metal walls and tops. The traps with wire or nylon mesh walls are cage traps (some with tops). All box and cage traps work under the same principle: an animal enters the trap through an opening, is usually attracted to bait, and moves a trigger or otherwise causes the door to close and lock. These traps are diverse and range in size from tiny boxes to capture mouse-sized mammals, to huge structures made of road culverts or logs to trap large carnivores, to netted cage traps to capture ungulates.
Box traps are used mostly to capture mammals weighing < 2 kg, although netted cage traps, culverts, and log traps capture large mammals. For researchers to build these latter traps requires considerable time, effort, and expense. Most netted cage traps are variations of Clover's (1954) trap: a pipe frame surrounded with netting ( McCullough 1975 ; VerCauteren et al. 1999 ). Barrel or culvert traps, made from ≥ 2 oil drums welded together or from sections of highway culvert, are commonly used to capture bears ( Ursus spp.). These traps are cumbersome to move and often are mounted on a trailer pulled by a truck. At best, they cannot be set far from roads. Large live-traps (1 x 1 x 2 m) built in situ with logs are also used to capture bears and other carnivores such as wolverines ( Gulo gulo;Copeland et al. 1995 ).
Animals captured in box and cage traps appear to undergo less trauma than those captured with limb-holding traps (Powell, unpublished data; White et al. 1991 ). Although some box traps hold mammals without injury for up to 24 hr ( Proulx et al. 1992 ), others may cause captured mammals to break teeth or to abrade skin on their muzzles (Powell and Proulx unpublished data on red squirrels [ Tamiasciurus hudsonicus ], muskrats [ Ondatra zibethicus ], weasels [ Mustela spp.], American martens [ Martes americana ], fishers [ Martes pennanti ], and black bears [ Ursus americanus ]). Box traps without insulated nest boxes and bedding are not recommended for small mammals in winter when temperatures are -20°C, or if researchers cannot check traps daily. Traps should be concealed and covered with vegetation to protect animals from sunlight and rain. Enclosures may be constructed to protect small traps from disturbance by predators ( Layne 1987 ). Remote devices can signal when a trap has sprung and allow quick attendance to captured animals, can reduce the accumulation of human scent at trap sites, and can reduce time and effort allocated to trap tending ( Arthur 1988 ). Remote devices must be constructed so that device failure causes traps to be checked.
Hancock and Bailey live-traps for beavers ( Castor canadensis ) resemble wire mesh suitcases that are set open, baited with appropriate food, and spring closed around a beaver that moves a trigger when taking the bait.
Pitfall Traps
A pitfall trap is a container (usually ≥ 40 cm deep and 20 to 40 cm in diameter; Jones et al. 1996 ), that has smooth vertical walls and is placed in the ground. Pitfalls are effective devices to capture the smallest (< 10 g) terrestrial mammals such as shrews ( Sorex spp., Blarina spp.; Spencer and Pettus 1966 ). Animals may be attracted to pitfall traps with bait or may fall into traps because they are placed along travel-ways or equipped with drift fences (barriers designed to direct small mammals into traps; Bury and Corn 1987 ; Handley and Kalko 1993 ). When set with bait or food, pitfall traps can maintain trapped mammals for several hours. Trapped mammals, however, may be subject to predation by larger mammalian predators and, when multiple shrews fall into the same trap, one often kills and consumes the others. Consequently, pitfall traps must be checked multiple times daily to maximize survival of trapped mammals.
Foothold Traps
Foothold traps have two jaws that open to 180° at set position and clamp together to hold an animal's paw. A trap is attached by a chain or cable to an anchor and restrains a captured animal from moving beyond the radius of the chain. Foothold traps are available in various models (some with padded jaws), with jaw spreads ranging from 7.6 cm for muskrats to 19 cm for wolves ( Canis lupus;Proulx 1999a ). These traps are used most frequently to trap carnivores, although diverse other species have also been targeted (e.g., Australian brushtail possums, Trichosurus vulpecula;Warburton et al. 1999 ). Most steel (unpadded) foothold traps, but not all, fail to meet Criterion II ( Proulx 1999a ). Skill in choosing trap size and in making and maintaining proper sets greatly affect the impact of a trap on the targeted mammal.
The EGG trap (EGG Trap Co., Ackley, Iowa) has a plastic housing with an interior pull trigger mechanism that causes a bar to prevent an animal from withdrawing its paw from the housing. It works well with mammals that manipulate and explore with their paws.
In the search for modified foothold traps that cause few injuries, Linhart et al. (1991) reported that 71% of coyotes ( n = 24) captured in steel foothold traps equipped with tranquilizing tabs (chlordrazepoxide and propiopromazine) sustained little or no visible foot damage. Sahr and Knowlton (2000) also reported minor injuries in wolves caught in traps with propiopromazine. Traps with tranquilizing tabs are not used by commercial trappers. Yet, along with recent developments in central nervous system depressants, the device may be of interest to researchers wishing to reduce further the injuries caused by padded-jaw traps.
Foot-, Neck-, and Body-Snares
A foot-snare holds an animal's paw in a loop of cable that is often tightened initially by a spring. Like foothold traps, foot-snares are usually anchored to restrain captured animals. Some, but not all, meet Criterion II ( Onderka et al. 1990 , Shivik et al. 2000 ). Although many different foot-snares are similar in appearance, they do not perform equally. Researchers must be familiar with the literature to select the foot-snares that work best for their animals.
Neck-snares have been used to capture canids ( Bjorge and Gunson 1989 ; Nellis 1968 ). Noonan (2002) promoted a new device that throws a loop of cable over an animal's head onto its neck. A stop attached to the cable prevents the loop from choking the captured animal, which is held as if restrained with a leash and collar. This device may cause fewer injuries than steel-jawed foothold traps, but it still does not meet Criterion II ( Shivik et al. 2000 ). Nonetheless, neck-snares equipped with diazepam tabs calmed captured coyotes and reduced facial and oral lacerations ( Pruss et al. 2002 ).
McKinstry and Anderson (1998) live-trapped beavers with body-snares instead of using heavy, cumbersome traps. Although entanglement and predation accounted for 5% mortality, the researchers effectively captured beavers around their chests, abdomens, or bases of their tails.
Corrals and Nets
A drive corral usually has nylon netting supported by posts to delineate an enclosure and a funnel fence that directs animals into the corral. Mammals as small as jackrabbits ( Lepus spp., Henke and Demarais 1990 ) and as large as ungulates have been restrained in drive corrals. Wolves ( Okarma and Jedrzejewski 1997 ) and ungulates ( Beasom et al. 1980 ) have been captured in nylon drive nets that are stretched loosely between two solid objects and supported by poles or branches. Drop nets can be effective to capture ungulates ( Conner et al. 1987 ); with baits or lures, animals are attracted under a drop net that is mechanically operated from a blind. Cannon nets, also called rocket nets, are large nets propelled over ungulates ( Beringer et al. 1999 ). A hand-held net-gun fired from a helicopter is a highly selective capture technique used for large ungulates ( Barrett et al. 1982 ; Carpenter and Innes 1995 ).
Drive corrals, nets, and string traps appear to improve efficiency over cage traps for trapping ungulates, but they have not been assessed from an animal welfare perspective. The preliminary data of Okarma and Jedrzejewski (1997) suggest that drive nets cause less injury to wolves than foothold traps.
Mist nests are effective for capturing flying bats ( Kuenzi and Morrison 1998 ). These loose nets, which are used mostly to live-trap birds, are made of thin, black line and are set between vertical poles. They are inexpensive and portable but must be tended constantly; bats become entangled and must be freed individually in a timely fashion ( Jones et al. 1996 ). The Hart trap, which has a large rectangular frame crossed by a series of vertical wires ( Constantine 1958 ; Tidemann and Loughland 1993 ; Tidemann and Woodside 1978 ), has an advantage over mist nets because it eliminates the tedious task of extracting each bat separately ( Jones et al. 1996 ). When a bat hits the bank of wires, it usually falls into a bag beneath the trap, from which it can be removed easily.
Dart Collars
Transponder radio collars with remotely triggered darts containing anesthetizing drugs cause less physical trauma and less stress than restraining traps (Powell, unpublished data). Because the animals are not restrained, however, the possibility exists (usually small) that the drug may take effect when an animal is in water, near a steep slope, or otherwise in a place that can result in serious injury when the animal falls.
Killing-Traps
Capture devices classified as killing-traps include snap and planar traps, rotating-jaw traps, killing box traps, killing-snares, pitfalls, and submarine traps. A brief description of each device appears below.
Snap and Planar Traps
Mousetraps and rat traps, often called snap traps, have one jaw (often "U"-shaped but sometimes a straight bar) that closes from 180° on a flat surface to strike an animal. In planar traps, the spring forms the killing bar. These traps range in size, and various models have been used for killing small mammals to medium-sized carnivores such as American martens ( Proulx 1999a , b ). Trap placement is critical, both to minimize the chances of trapping nontarget animals that are too large to be killed quickly by the snap trap used and to maximize the chances that an animal approaches a trap so that the jaw can give a killing blow. Despite being used "forever" and having killed uncountable numbers of small mammals (and birds), effectiveness has been quantified for only a very few snap traps ( Proulx 1999a , b ). Of these, the Bionic trap (W. Gabry, Vavenby, British Columbia, and A. Gabry, Camrose, Alberta), a large mousetrap powered by a coil spring, appears to be the most versatile trap to meet Criterion I, and it has been found effective for the capture of weasels (Proulx unpublished data), minks ( Mustela vison ), martens, and fishers ( Proulx 1999a ).
The Fenn trap, developed in Britain during the 1950s, is a large snap trap used to capture small mustelids. It may be judged more humane than foothold traps because fewer animals are found alive or seriously injured ( King 1975 ). Nevertheless, on the basis of limited field observations and on laboratory mechanical evaluations (Proulx unpublished data; Von Eerdenburg 1988 ), Fenn traps (and similar models) appear not to meet Criterion I (Fenn traps, FHT Works, High Street, Astwood Bank, Redditch, Worchestershire, England).
One planar trap, the Kania 2000 trap (Kania Industries Ltd., Nanaimo, British Columbia), is appropriate for mammals that are the size of American martens; it met Criterion I in laboratory tests but had equivocal results in the field ( Proulx 1999a ). The trap was being sold in Europe in 2002 for the capture of Norway rats ( Rattus norvegicus ) and gray squirrels ( Sciurus carolinensis ) (C. Kania, Kania Industries Ltd., Nanaimo, British Columbia, personal communication, 2003).
Rotating-Jaw Traps
A rotating-jaw trap has two metal frames, usually square or rectangular and hinged at the centers of two opposite sides, which allow a torsion spring on each side to rotate the frames and close them in a scissor-like fashion. Rotating-jaw traps, although called back-breaking traps, do not always kill animals quickly and sometimes act more like holding devices ( Novak 1981 ). Proulx's (1999a) review of the most popular models noted that standard rotating-jaw traps do not meet Criterion I. By welding clamping bars on the striking jaws (to increase clamping force), using stronger springs (to increase striking force), and using triggers that position animals properly for blows in vital regions, rotating-jaw traps can meet Criterion I. Researchers must select rotating-jaw traps carefully. Many models available on the market bear similar names but do not all meet Criterion I ( Table 2 ).
Characteristics of rotating-jaw trap models and their ability to kill mammals (after Barrett et al. 1989 ; Fur Institute of Canada 1994 ; Gilbert 1992 ; Novak 1981 ; Proulx 1990 ; Proulx and Barrett 1993a , b ; Proulx and Drescher 1994 ; Proulx et al. 1990 , 1995b ; Sabean and Mills 1994 ) a , b
| Trap model . | Mean momentum (kg/m/sec) . | Range of clamping forces (N) . | Species . | Humaneness * . |
|---|---|---|---|---|
| C120 Magnu | 1.09 | 254-473 | American marten, mink | Yes |
| C330 with clamping bars | 3.27 | 271-607 | Lynx, beavers | Yes |
| Conibear 330 | 2.65 | 0-252 | Lynx, raccoon, beaver | No |
| Conibear 160 | ? | ? | American marten, raccoon | No |
| Conibear 220 | 1.45 | 206-472 | Fisher, raccoon | No |
| Conibear 280 | 2.03 | 0-364 | Raccoon | No |
| Conibear 120 | 0.54 | 0-224 | American marten | No |
| Sauvageau 2001-5 | 0.81 | 310-430 | American marten | No |
| Sauvageau 2001-8 | 2.10 | 446-585 | Raccoon Arctic fox | No Yes |
| Trap model . | Mean momentum (kg/m/sec) . | Range of clamping forces (N) . | Species . | Humaneness * . |
|---|---|---|---|---|
| C120 Magnu | 1.09 | 254-473 | American marten, mink | Yes |
| C330 with clamping bars | 3.27 | 271-607 | Lynx, beavers | Yes |
| Conibear 330 | 2.65 | 0-252 | Lynx, raccoon, beaver | No |
| Conibear 160 | ? | ? | American marten, raccoon | No |
| Conibear 220 | 1.45 | 206-472 | Fisher, raccoon | No |
| Conibear 280 | 2.03 | 0-364 | Raccoon | No |
| Conibear 120 | 0.54 | 0-224 | American marten | No |
| Sauvageau 2001-5 | 0.81 | 310-430 | American marten | No |
| Sauvageau 2001-8 | 2.10 | 446-585 | Raccoon Arctic fox | No Yes |
See text for all references.
Sabean B, Mills J. 1994 . Raccoon: 6″ × 6″ body-gripping trap study., Unpublished 3-page report prepared by the Nova Scotia Department of Natural Resources in 1994.
At a 95% confidence, ability to render ≥70% of animals unconscious in <3 min.
Characteristics of rotating-jaw trap models and their ability to kill mammals (after Barrett et al. 1989 ; Fur Institute of Canada 1994 ; Gilbert 1992 ; Novak 1981 ; Proulx 1990 ; Proulx and Barrett 1993a , b ; Proulx and Drescher 1994 ; Proulx et al. 1990 , 1995b ; Sabean and Mills 1994 ) a , b
| Trap model . | Mean momentum (kg/m/sec) . | Range of clamping forces (N) . | Species . | Humaneness * . |
|---|---|---|---|---|
| C120 Magnu | 1.09 | 254-473 | American marten, mink | Yes |
| C330 with clamping bars | 3.27 | 271-607 | Lynx, beavers | Yes |
| Conibear 330 | 2.65 | 0-252 | Lynx, raccoon, beaver | No |
| Conibear 160 | ? | ? | American marten, raccoon | No |
| Conibear 220 | 1.45 | 206-472 | Fisher, raccoon | No |
| Conibear 280 | 2.03 | 0-364 | Raccoon | No |
| Conibear 120 | 0.54 | 0-224 | American marten | No |
| Sauvageau 2001-5 | 0.81 | 310-430 | American marten | No |
| Sauvageau 2001-8 | 2.10 | 446-585 | Raccoon Arctic fox | No Yes |
| Trap model . | Mean momentum (kg/m/sec) . | Range of clamping forces (N) . | Species . | Humaneness * . |
|---|---|---|---|---|
| C120 Magnu | 1.09 | 254-473 | American marten, mink | Yes |
| C330 with clamping bars | 3.27 | 271-607 | Lynx, beavers | Yes |
| Conibear 330 | 2.65 | 0-252 | Lynx, raccoon, beaver | No |
| Conibear 160 | ? | ? | American marten, raccoon | No |
| Conibear 220 | 1.45 | 206-472 | Fisher, raccoon | No |
| Conibear 280 | 2.03 | 0-364 | Raccoon | No |
| Conibear 120 | 0.54 | 0-224 | American marten | No |
| Sauvageau 2001-5 | 0.81 | 310-430 | American marten | No |
| Sauvageau 2001-8 | 2.10 | 446-585 | Raccoon Arctic fox | No Yes |
See text for all references.
Sabean B, Mills J. 1994 . Raccoon: 6″ × 6″ body-gripping trap study., Unpublished 3-page report prepared by the Nova Scotia Department of Natural Resources in 1994.
At a 95% confidence, ability to render ≥70% of animals unconscious in <3 min.
Killing Box Traps
Killing box traps have a striking jaw set within a box or pipe (i.e., the traps are driven by a spring to strike an animal ventrally when the trigger is released). These traps are used in North America mostly to kill pocket gophers ( Thomomys spp.). Most have not been tested for effectiveness, but two commercially available traps did fail to meet Criterion I and one experimental design did meet the criterion ( Proulx 1999c ).
Killing-Snares
Manual snares are wire nooses set on land or under water; an animal captured provides the energy to tighten the noose around its own neck. Tests of manual snares with several target species of terrestrial mammals and with beavers failed to meet Criterion I ( Proulx 1999a ). Power snares, wherein one or more springs provide the energy necessary to tighten the noose, are commercially available to kill large carnivores but require further development to meet Criterion I. Proulx and Barrett (1990) showed that some models have the potential to render neck-captured red foxes ( Vulpes vulpes ) unconscious in ≤ 6 min.
Pitfall Traps
Pitfall traps with water in the bottom are often used to capture and to drown shrews and other small mammals. Specimens are not damaged by capture, multiple captures are possible, and traps do not require frequent monitoring. Drowning of some small mammals (e.g., meadow voles, Microtus pennsylvanicus ), however, does not occur consistently within 3 min ( Proulx 1999a ). In addition, killing trapped animals by drowning raises ethical issues because drowning animals often die slowly with hypoxia-induced discomfort and distress (e.g., Bluett 2001 ; Ludders et al. 1999 ).
Traps Used in Drowning Sets
Submarine traps (box traps set under water, or rotating-jaw or foothold traps set on surface and sliding under water when fired) are used traditionally to capture beavers, muskrats, minks, and otters ( Lontra canadensis ). Because captured animals struggle for more than 3 min ( Proulx 1999a ), these traps do not meet Criterion I, and the traps raise ethical concerns related to drowning.
Sets
All traps can be set in diverse ways. Researchers consistently adapt their sets to increase trapping efficiency and selectivity and to increase trap effectiveness. Proper sets can sometimes make the difference between a trap meeting and not meeting Criterion I or II. When opting for a particular trap set, researchers must consider (1) trap elements, (2) trap location, and (3) baited versus trail sets.
Trap Elements
For traps to be most effective and selective, the tripping force of the trigger must match the size of the target animals. A trap with a heavy tripping weight cannot trap small target animals effectively, and a trap with a light tripping force may not be selective enough (e.g., Phillips and Gruver 1996 ; Smith et al. 1971 ). Trigger characteristics (e.g., shape and size) may discourage an animal from entering a trap. For example, while Barrett et al. (1989) showed that C120 Magnum (Les Pièges du Québec Enr., St. Hyacinthe, Québec) traps captured and killed martens effectively and met Criterion II, Naylor and Novak (1994) reported poor capture success. The latter had equipped their traps with four-prong triggers that discouraged animals from entering the traps.
All of the elements of a trap must be taken into consideration when setting it. For example, Mowat et al. (1994) found that foot-snares attached loosely to trees or drag poles led to major injuries that could cause the death of the animals in nearly 30% of all captures. In contrast, traps properly set to eliminate tangling of the snare in brush caused major injuries in only 3% of captures. In culvert traps, ventilation holes of the wrong size lead bears to break teeth. If traps are not elevated and outfitted with sufficient drain holes, trapped bears stew in their own urine and feces.
Trap Location
No matter how humane a trap might be, placing it in an improper location can lead to unacceptable capture results. For example, culvert traps made from black oil drums can be effective bear traps but they become solar ovens in direct sunlight. Raccoons ( Procyon lotor ) captured in EGG traps along water courses ( Hubert et al. 1996 ) may not sustain injuries but may suffer or even die from hypothermia if they are held standing in cold, shallow water.
The position of a trap in a set has a major impact on success of capture and injuries sustained by captured animals. For example, when trapping lynxes ( Lynx canadensis ) with a killing, rotating-jaw trap, Proulx et al. (1995b) placed the trap at least 23 cm above ground with its center in line with the bait attached at the back of a cubby (logs piled in a funnel shape to direct target animals to the bait and trap). When traps were set too low, lynxes tried unsuccessfully to go over the trap or lost interest in the bait. When traps were too high, lynxes used their paws to reach the bait and inadvertently fired the trap, thus becoming restrained by their paws instead of being killed by blows to their necks.
Bait Versus Trail Sets
A baited set uses food or scent to draw target animals to the trap, whereas a trail, or blind, set is placed where a target animal is expected to travel on its own. Baited traps have higher capture rates than trail sets, particularly in carnivores, but attract nontarget as well as target animals. Capturing nontarget animals may therefore lower the efficiency of baited traps. Trail sets may be highly efficient and selective for capturing animals that establish trails (e.g., muskrats, hares [ Lepus spp.], bears, and deer [Cervidae]).
Trapping Methods for Specific Mammals
Small Mammals
Small mammals are herbivorous or insectivorous mammals generally weighing ≤ 300 g (i.e., the size of a squirrel or smaller). Some researchers have considered rabbits and hares to be small mammals (ca. 1 kg), while others have considered squirrels not to be. The category generally includes, but is not limited to, small rodents, small lagomorphs, insectivores, bats (sometimes), and small marsupials.
For most research, these mammals are best live-trapped using box or cage traps (e.g., Bondrup-Nielsen 1987 ; Getz et al. 2001 ; Gilbert and Krebs 1991 ; Millar and Innes 1983 ; Peacock and Smith 1997 ; Steele and Powell 1999 ; Stratham and Harden 1982 ; Wolff and Cicirello 1991 ) or pitfalls for shrews (e.g., Spencer and Pettus 1966 ). Because of their high metabolic rates, these mammals (especially shrews) cannot remain in live-traps a long time without food, therefore bait must be of an adequate amount to last between trap checks and must nourish the target animals appropriately. During winter, bedding in live-traps can reduce mortalities; raw wool with natural lanolin makes excellent, warm, dry bedding because it is an excellent insulator that repels water. Live-traps for small mammals usually need to be checked at least twice a day, and more often for shrews or when weather is extremely hot, cold, or wet. Sometimes making live-traps inoperative during the heat of the day is best. Checking traps at night may be warranted but only when animals can be handled safely and effectively. Traps must be set to protect captured animals from flooding and sometimes from harassment by predators.
When research design requires mammals to be killed, snap traps are generally used ( Batzli et al. 1983 ; Krefting and Ahlgren 1974 ; Powell and Brooks 1981 ). Because most of these traps have not been tested against Criterion I, care should be taken to use traps that will kill target animals quickly, to use sets that encourage target mammals to approach from a direction that makes the traps most effective, and to use sets that discourage nontarget animals.
Trap design should be appropriate to the target mammals and to the goals of the research. Points to consider include trap material (e.g., wooden live-traps provide extra insulation but are bulky; collapsible, sheet metal traps can be carried far into back country but are not as sturdy as noncollapsible traps), construction (e.g., whether it allows multiple captures), and habits of target mammals. Different trap designs and constructions, for example, have different trapping efficiencies for different small mammal species, mammals of different mass, and, possibly, different study sites ( Boonstra and Rodd 1982 ; Slade et al. 1993 ).
Traps should be spaced appropriately to answer the research questions and appropriately for the biology of the mammals being studied. Traps might be spaced so that each individual has one trap within its home range (to trap as many different individuals as possible) or so that each individual has many traps in its home range (to recapture each individual many times and delineate its home range). Traps are commonly set along transects or on grids ( Sullivan 1997 ). Traps should be placed at habitat features (e.g., in coarse woody debris, by trees, along runways, or by burrows) when possible to maximize capture success. Setting two or more traps at every station reduces saturation of traps with "trap-happy" individuals (live-traps) or with individuals that are readily captured (killing-traps; Drickamer 1987 ). Trap numbers and placement are ultimately a balance of research design and the number of traps that can be tended feasibly ( Bowman et al. 2001 ).
Pitfall traps must be deep enough to prevent escape of target mammals. They should be placed to minimize capture of nontarget animals and to minimize loss to predators. For some species, guide fences (natural objects or constructed) increase capture efficiency. Pitfall traps are also often set along transects or on grids. To trap fossorial mammals, traps must be set in their burrow systems, which are often identified by clusters of relatively fresh mounds ( Witmer et al. 1999 ).
Mist nests and Hart traps for bats must be tended constantly to prevent bats from becoming inextricably entangled, to prevent them from chewing the net, to prevent escape, and to free the bats in a timely fashion ( Jones et al. 1996 ; Kuenzi and Morrison 1998 ). Weather conditions, habitat, moonlight, roosting habits of the target bat species, daily and nightly activity patterns, seasonal movements, and colony size all can influence capture success and should be considered in capture protocols ( Jones et al. 1996 ).
Medium-sized Herbivores
Medium-sized herbivorous mammals generally weigh between 300 g and 35 kg. The category includes a diversity of rodents, lagomorphs and marsupials. They are commonly captured in box or cage traps ( Grisemer et al. 1999 ; Proulx and Gilbert 1983 ; Sullivan 1997 ).
Hancock and Bailey live-traps for beavers are bulky and usually set in shallow water ( Hodgdon 1978 ; Taber and Cowan 1969 ). The traps can be difficult to set, and care must be taken to prevent a trapped beaver from rolling its trap into deep water and drowning. McKinstry and Anderson (1998) used body-snares to live-trap beavers. Trap mortality with body-snares, due to predation and entanglement of animals, was slightly higher than those reported for traps.
Henke and Demarais (1990) used a drive corral to capture black-tailed jackrabbits ( Lepus californicus ). With help from assistants, Henke and Demarais drove jackrabbits toward a funnel of fencing that led to a 4.6 x 3.7 m corral. A gill net inside the corral improved safety and handling efficiency. They judged the drive corral to be safer and to provide easier handling than cage traps, which are most often used for rabbits and hares.
Medium-sized marsupials are often trapped using cage traps (e.g., Fisher and Lara 1999 ). Warburton et al. (1999) trapped Australian brushtail possums using padded and unpadded foothold traps and cage traps. Possums captured in unpadded foothold traps experienced the greatest physical injury, whereas possums captured in cage traps showed the mildest physiological and behavioral responses. Warburton and colleagues did not report relative capture efficiencies.
A variety of killing-traps are available for medium-sized herbivores because many of these mammals are trapped for fur or meat. Rotating-jaw traps and drowning foothold sets are commonly used to trap semiaquatic mammals ( Gilbert 1992 ; Parker 1983 ). Although they do not meet Criterion I, manual neck-snares are used to capture snowshoe hares ( Proulx et al. 1994 ).
Although traps for medium-sized herbivores are sometimes set along transects or grids ( Sullivan 1997 ), trapping is usually most productive when traps are set at specific habitat features such as feeding stations, defecation posts, beaver dams, runways close to active houses and burrow systems, and trails ( Griesemer et al. 1999 ; Proulx 1981 ; Proulx and Gilbert 1983 ). Baker and Clarke (1988) live-trapped nutrias, or coypus ( Myocastor coypus ), on baited rafts.
Many animals use runways in common with snowshoe hares ( Keith and Meslow 1966 ). In Newfoundland, snaring for hares has affected the endangered American marten population significantly, and snares must be modified to capture hares but to allow martens to escape ( Proulx et al. 1994 ).
Large Herbivores
The category of large herbivores includes deer, sheep, and goats (Capridae), antelopes (Bovidae), other ungulates, and kangaroos (Macroporidae). These animals are often captured using netted cage traps, rocket or cannon nets, corral traps, or drive nets ( Aldous 1958 ; Beasom et al. 1980 ; Beringer et al. 1999 ; Clancy and Croft 1992 ; Hansen et al. 1980 ; Spillett and Zobell 1967 ; VerCauteren et al. 1999 ).
Animals must be handled quickly, and possibly immobilized once captured, to minimize self-inflicted injuries from struggling and to reduce capture myopathy. Capture myopathy, or capture stress, is a disease primarily of ungulates (but also many other mammals and birds). It is associated with capture and handling, and it results in cardiac and skeletal muscle necrosis ( Pond and O'Gara 1994 ; VanReenen 1980 ). Collapsible netted cage traps facilitate handling ( VerCauteren et al. 1999 ). Beringer et al. (1999) recommended limiting noise at capture sites, minimizing handling times, and blindfolding animals. Immobilizing deer chemically reduces struggling and physical injuries. Capture myopathy is sometimes related to specific capture methods within studies but not across studies ( Beringer et al. 1999 ; Kock et al. 1987 ; Read et al. 2000 ), leading us to believe that details related to capture methods are critical to this disease. Mortality due to predators and accidents may be highest with netted cage traps ( Beringer et al. 1999 ).
Captures with rocket nets and netted traps are most effective at prebaited sites ( Beringer et al. 1999 , VerCauteren et al. 1999 ). Trapping devices should be placed in areas that receive high use by animals. Trails between bedding and feeding areas are ideal sites if they are far enough from roads so that neither traps nor animals are visible to the public ( VerCauteren et al. 1999 ). Brown or green netting is less obvious to people ( Rongstad and McCabe 1984 ). To minimize mortality and injury, netted cage traps should be checked at least twice a day or monitored remotely. Because rocket nets are fired when researchers choose, researchers can be more selective than with other traps. Injuries and mortality can be reduced by building corral traps with wooden posts (not metal, nylon, or other cloth netting; and not woven wire; Hansen et al. 1980 ). Corral traps and drive nets can capture larger numbers of animals at one time.
Moose ( Alces alces ) and other large ungulates have been captured highly selectively using a hand-held net-gun fired from a helicopter ( Barrett et al. 1982 ; Carpenter and Innes 1995 ). White-tailed deer ( Odocoileus virginianus ) have been recaptured using radio telemetric collars outfitted with darts ( Mech et al. 1984 ).
Small to Medium-sized Carnivores and Omnivores
Small to medium-sized mustelids, procyonids, small felids and canids, herpestids and small vivirrids, and dasyurids and didelphids can all be trapped effectively with box or cage traps baited with food or attractive scents ( Arthur 1988 ; Bluett 1992 ; Bull et al. 1996 ; Genovesi and Boitani 1997 ; Jones 1995 ; Jones and Barmuta 1998 ; Powell 1979 , 1993 ; Stratham and Harden 1982 ; Woolf and Nielsen 2002 ). Many of these mammals (particularly mustelids) struggle with cage traps, damage teeth and claws, and scrape skin from their muzzles if mesh size is too large (mesh size should be ≤ 2.5 cm, and ≤ 1 cm for the smallest of these mammals).
If possible, weasels and minks are best trapped in wooden traps, preferably with an attached, insulated nest box. A wooden nest box is also good for American martens, even attached to a cage trap. Fishers usually pull branches and ground debris into cage traps once captured but seldom struggle with the wire mesh until approached by a human; thereafter, struggle with the wire mesh may be vigorous and can lead to significant, self-inflicted injury (Powell, unpublished data). Raccoons and oppossums ( Didelphis virginiana ) can be captured in box traps and EGG traps with little to no injury ( Hubert et al. 1996,1999 ; Proulx et al. 1993b ). Raccoons should not be captured in conventional foothold traps because they may mutilate themselves once captured ( Hubert et al. 1996 ; Proulx et al. 1993b ).
Although felids have been considered reluctant to enter cage traps, Woolf and Nielsen (2002) reported that bobcats ( Lynx rufus ) were twice as easy to capture in cage traps versus foothold traps. Trap injuries were uncommon in cage traps and included only minor cuts or bruises. Radio monitoring revealed that bobcats resumed their normal activities within 24 hr.
Canids appear truly reluctant to enter cage traps. They, felids, and the largest mustelids can be trapped with foothold traps and foot-snares, which must be matched in size to the target species to minimize injuries. Properly chosen foothold traps, especially padded traps, may meet Criterion II ( Proulx 1999a ; Seddon et al. 1999 ). Long-spring number 1½ foothold traps set for arctic foxes ( Alopex lagopus ) meet Criterion II if checked daily ( Proulx et al. 1994 ). Many foothold traps with padded jaws meet Criterion II to capture other foxes ( Vulpes spp.), coyotes ( Canis latrans;Onderka et al. 1990 ; Phillips et al. 1996 ), bobcats ( Olsen et al. 1988 ), and otters ( Serfass et al. 1996 ).
The Åberg (Nordic Sport AB, Skellefteå, Sweden) and Fremont (Fremont Humane Traps, Beaumont, Alberta) snares can capture canids without causing serious injuries ( Englund 1982 ; Onderka et al. 1990 ), and the Fremont ( Mowat et al. 1994 ) and Schimetz-Aldrich (D. Schimetz, Sekiu, Washington) ( Logan et al. 1999 ) snares also meet Criterion II for felids. The Belisle and the Wildlife Service snare systems used to capture coyotes appear not to meet Criterion II ( Shivik et al. 2000 ).
Sets that minimize capture of nontarget animals must be developed. Nontarget animals, especially those smaller than the target species, can be severely injured in foothold traps. Traps set for small carnivores must be anchored well enough to withstand capture by large, nontarget carnivores; otherwise, a large carnivore may escape carrying a small trap on its paw.
Mustelids can be kill-trapped in powerful snap traps (e.g., the Bionic), in planar traps (e.g., the Kania 2000 ), and in rotation-jaw traps (e.g., C120 Magnum), which meet Criterion I. Arctic foxes and lynxes can be killed effectively in the Sauvageau 2001-8 (Les Pièges du Québec Enr.) and the modified C330, respectively, which meet Criterion I ( Proulx 1999a ). Other small to medium-sized carnivores can be trapped with the EGG trap, foothold traps, or foot-snares and killed humanely when found in traps. Sometimes medium-sized carnivores can be killed humanely by sharp-shooting ( Kreeger et al. 1990 ).
Researchers experienced with the natural history of the mammals they study recognize potential trapping sites. The presence of scats or tracks often call for placing a trap. Special habitat features such as snags, coarse woody debris, nearby dens, squirrel middens, snowshoe hare trails, and proximity to water usually provide researchers with successful trapping sites ( Buskirk and Powell 1994 ; Hubert et al. 1996 ; Woolf and Nielsen 2002 ). For some research designs, superimposing a grid of appropriate cell size on a map of the study area and putting a trap in appropriate sites in each cell provides good sampling.
Large Carnivores and Omnivores
The category of large carnivores and omnivores includes the large felids, hyaenids, canids, and ursids. Large felids (e.g., mountain lions [ Puma concolor ] and tigers [ Panthera tigris ]) can be captured in foot-snares without serious injuries ( Goodrich et al. 2001 ; Logan et al. 1999 ). Wolves, coyotes, and other large canids are most often trapped with foothold traps or foot-snares ( Bjorge and Gunson; 1989 , Onderka et al. 1990 ). Chosen and used properly, foothold traps set for large canids and felids can meet Criterion II. Neck-snares with tranquilizing tabs have been used for coyotes ( Pruss et al. 2002 ), and drive nets have been used for wolves with less injury than foothold traps ( Okarma and Jedrzejewski 1997 ). Although drive nets may not be practical for many studies, they should not be summarily dismissed without being investigated as an option. Multiple capture cage traps set at den entrances can be effective for capturing coyote pups ( Foreyt and Rubenser 1980 ). Wolves and other large mammals have been recaptured using radio telemetric collars outfitted with darts ( Mech et al. 1984 ; Powell et al. 1997 ), and this capture appears to meet Criterion II when used for black bears (Powell, unpublished data).
Brown hyaenas ( Hyaena brunnea ) can be captured using cage traps ( Mills 1990 ), and spotted hyaenas ( Crocuta crocuta ) using darts fired from specially built air rifles ( Frank 1986 , Mills 1990 ). Care must be taken with these firearms to hit a large muscle mass with the dart to avoid serious injury.
Bears are trapped in cumbersome but effective barrel and culvert traps ( Graf et al. 1992 ). Most culvert traps have a heavy guillotine door that drops when a bear pulls bait from a trigger. A door can kill a cub following its mother into a trap. Because each trap is made individually and is different from others, each must be compared separately with Criterion II. One barrel trap used by Powell (unpublished data) to trap black bears met Criterion II, but capture of a bear in another barrel trap caused extensive tooth breakage. These traps are difficult to transport and cannot be distributed effectively across most back-country study areas.
Bears can also be captured effectively in foot-snares modified with an automobile hood spring for cushioning ( Graf et al. 1992 ; Huber et al. 1996 ; Johnson and Pelton 1980 ; Powell et al. 1997 ), which can be transported far into back country, allowing effective sampling in remote study areas. Bears captured in foot-snares often struggle energetically and much more than bears in barrel traps. If branches, logs, or small trees tangle in the snare cable, cushioning devices may not work and bears may break bones or the cable may cut into the captured paw. Otherwise, common trap injuries rarely extend beyond swelling and minor cuts or abrasions. Foothold snares used by Powell (unpublished data) met Criterion II; most bears were physically exhausted but had no long-term effects. Small bears captured in snares can be, unfortunately, subject to predation by large bears. Consequently, where bear densities are high and big bears frequent traps, foot-snares may not be tenable.
In US states where hunting with dogs is legal, researchers have hired hunters and their dogs to tree black bears and mountain lions, where the animals can be drugged ( Hornocker 1970 ; Ruggles 2002 ; Seidensticker et al. 1973 ). These predators may become physically exhausted, and drugged animals can be injured when being lowered from trees, yet the technique has not been evaluated relative to Criterion II.
Baited sets strategically placed along trails and watercourses may be more effective than blind sets to capture large carnivores and omnivores that are naive. These mammals are highly intelligent, however, and trap-wise carnivores and omnivores can be extremely difficult to recapture and often require trail sets that bear no human scent.
For studies requiring animals to be killed, these large carnivores and omnivores can be live-trapped and killed humanely once captured. Sometimes they can be killed humanely by sharp-shooting.
Marking Mammals
For most research on wild mammals, individuals that have been trapped must be identifiable on recapture or from a distance. Tasks such as estimating population sizes, calculating demographic variables, and discerning behavior of individuals all require that individual animals be identifiable. Use of natural marks is preferred where feasible, although lack of obvious marks, secretive behavior, and dense habitat usually preclude using natural marks to identify mammals.
When marks are applied, whether temporary or permanent, they should be as painless as possible and should not affect the animals' behavior or health ( ASM/ACUC 1998 ). Marks must be matched to research objectives and must be appropriate for the mammals' sizes, future growth, body shapes, and behavior. A variety of short-term, long-term, and permanent markers are available, some of which have been evaluated specifically for their effects on research animals. Researchers should carefully investigate the use of anesthetic drugs to facilitate handling.
Short-term Markers
Short-term markers usually persist less than a year. They include marks lost during subsequent molts, nocturnal lights, chemical products that are shed after short periods, and body attachments.
Fur clipping and dyeing. One can mark small mammals temporarily by shaving unique patterns in the hair on their backs. This technique is particularly useful to mark shrews that cannot be ear-tagged ( Sullivan 1997 ). Dyed hair can be used to mark mammals of all sizes in the same way ( Hanks 1969 ; McCracken 1984 ; Ramsay and Stirling 1986 ; Shriner and Stacey 1991 ; Singer 1978 ). Dyes are particularly useful for mammals of light pelage, although the range of colors is small. Yellow picric acid and pink Rhodamine B have been used to mark lagomorphs ( Brady and Pelton 1976 ; Keith et al. 1968 ), mountain beavers ( Aplodontia rufa;Lindsey 1983 ), and other animals ( Fisher 1999 ). The effects of changing a mammal's hair coat are unknown and may alter cryptic coloration and hence predation, thermoregulation, and a mammal's ability to deal with weather and its physical environment.
Nocturnal lights. "Pinlights" and "flashers" taped to the fur of nocturnal mammals or to collars allow researchers to follow them ( Barbour and Davis 1969 ; Batchelor and McMillan 1980 ; Carpenter et al. 1977 ). The duration and intensity of the markers depend on the size and life span of the batteries.
Powders. Fluorescent powders are used to detect the presence and movements of small mammals ( Lemen and Freeman 1985 ; Jike et al. 1988 ; Proulx et al. 1995a ). The fluorescent powder trails left by mammals are detected by portable, ultraviolet lights. Reliability of detection varies with vegetation cover and precipitation ( Mullican 1988 ).
Body attachments. Streamers and colored disks of different lengths and color codes attached to a mammal's body or to eartags allow identification of animals from a distance ( Aldous and Craighead 1958 ; Daan 1969 ; Knowlton et al. 1964 ; Lentfer 1968 ; Queal and Hlavachick 1968 ).
Punch-marking. old> A tattoo instrument normally used to mark domestic livestock can punch small holes in unique patterns (numbers) through the outstretched wing membranes of bats ( Bonaccorso and Smythe 1972 ). Punch marks remain legible for only about 5 mo ( Bonaccorso et al. 1976 ).
Long-term Markers
Long-term markers include eartags, collars and bands, passive integated transponder (PIT 1 ) tags, radioactive markers, and beta lights. Brief descriptions of markers in this category appear below.
Eartags. Eartags made from metals or plastics of all shapes, sizes, and colors and stamped with codes are the mark of choice for many mammals ( Proulx and Gilbert 1983 ; Smith and Gao 1991 ; Steigers and Flinders 1980 ; Stirling 1989 ; Sullivan 1997 ). Fingerling ear tags have been used to mark bats since the 1930s ( Mohr 1934 ) but are not suitable for large-eared bats that exhibit rapid ear movements synchronized with echolocation ( Stebbins 1978 ). Eartags can also be applied to interdigital webbing ( Keith et al. 1968 ), to the outer toes of the hind feet ( Linduska 1942 ), or to the skin of mammals' backs ( Errington and Errington 1937 ).
Eartags should be loose enough not to interfere with blood circulation, and puncture marks should be treated appropriately to prevent infection and ensure healing ( Nietfeld et al. 1994 ). Eartags can be pulled out by animals grooming each other ( Stirling 1989 ) or can catch on vegetation (Proulx, unpublished data). Turning metal, crimping eartags in a mammal's ear so that the clasp is outermost appears to minimize loss (Powell, unpublished data). Using redundant marking overcomes problems from tag losses. Eartags may inhibit grooming and lead to infestations of mites and ticks (e.g., Ostfeld et al. 1996 ).
Collars and bands. Neck collars, fixed in size or expandable for growing animals, have been used to mark many mammal species ( Beale and Smith 1973 ; Hawkins et al. 1967 ; Rudge and Joblin 1976 ). Neck collars outfitted with radio transmitters allow identification of individual animals and their movements. Neck collars must be sized carefully to be loose enough not to cause injury or skin irritation. Wing bands or bead-chain necklaces are best for bats ( Barclay and Bell 1988 ). Researchers must be familiar with the many special requirements for bands and necklaces on bats ( Barclay and Bell 1988 ; Bonaccorso et al. 1976 ; Handley et al. 1991 ; Kunz 1996 ).
PIT tags. Pit tags provide permanent identification. Each tag consists of an electromagnetic coil and custom-designed microchip that emits an analog signal when excited by electromagnetic energy from a scanning wand. The transponder chip is uniquely programmed with an alpha or numeric code, and > 34 billion combinations are available ( Nietfeld et al. 1994 ). Once inserted under a mammal's skin with a large bore syringe, a PIT tag can be "read" by a scanner. PIT tags are expensive, however, relative to most other marking methods, and they require a specific scanner matched to the tag type to read the identification. PIT tags may wander under an animal's skin, especially on large mammals. Nonetheless, PIT tags may be more reliable than eartags on some small mammals ( Harper and Batzli 1996 ; Williams et al. 1997 ).
Radioactive markers. A variety of mammals have been marked with radioisotopes as inert implants, external attachments, and metabolizable radionucleoides ( Linn 1978 ; Nellis et al. 1967 ; Pelton and Marcum 1975 ). When using radioactive tags, researchers must follow established federal safety standards.
Betalights. A betalight is a phosphor-coated glass capsule containing a small quantity of mildly radioactive tritium gas. When the phosphor is struck by low-level beta radiation from tritium, it produces visible light of a characteristic color ( Rudran 1996 ). Betalights can be incorporated with other markers ( Cheeseman and Mallinson 1980 ; Davey et al. 1980 ; Hardy and Taylor 1980 ). They appear to pose no appreciable health hazard due to radiation and may function for years ( Rudran 1996 ).
Permanent Markers
Permanent markers include natural markings, mutilations, freeze branding, and tattoos. Brief descriptions of the markers in this category appear below.
Natural markings. The size, shape, or peculiarities of, for example, natural body marks, horn characteristics, and scars may be used to identify individual mammals ( Kelly 2001 ; Mukinya 1976 ; Pennycuick 1978 ).
Mutilations. Toe clipping, where the claw and first joint of the toe are removed with dissecting scissors, is an inexpensive, rapid, and permanent marking technique ( Blair 1941 ). This technique is suitable for small mammals when no other marking methods are appropriate ( ASM/ACUC 1998 ). Toe clipping is judged unethical by some researchers (e.g., Sullivan 1997 ) and may ( Pavone and Boonstra 1985 ) or may not ( Montgomery 1985 ) decrease the life span of some small mammals. It may, however, be less detrimental than eartags and other marks for other small mammals ( Ostfeld et al. 1996 ). It is not recommended for bats ( Barclay and Bell 1988 ).
Ears may be punched or clipped in a variety of coded systems ( Blair 1941 ; Honma et al. 1986 ; Kruuk 1972 ). Punched holes or slits cut into foot webs have been used to mark beavers ( Aldous 1940 ) and nutrias (coypus; Davis 1963 ). Natural holes and cuts in ears can sometimes be confused for punched holes, making these marks not unique for some mammals (e.g., Peromysucs spp. and black bears; Powell, unpublished data).
Freeze branding. Freeze branding, or cryobranding, applies either a copper branding iron that is supercooled in liquid nitrogen, a mixture of dry ice and alcohol, or a commercial refrigerant to an area of the body ( Hadow 1972 ). Performed correctly, freeze branding kills the pigment-producing melanocytes of the skin but not the hair follicles, so the hair and skin that grow back in the branded area are permanently white ( Day et al. 1980 ; Hadow 1972 ). Freeze branding can produce diverse, unique marks ( Hadow 1972 ; Newsom and Sullivan 1968 ; Pfeifer et al. 1984 ; Rood and Nellis 1980 ; Sherwin et al. 2002 ); proper timing of a freeze brand varies with animal species, however, and producing dependable brands requires experience.
Tattoos. A tattoo, applied with special pliers or an electric tattooing pencil, is a series of tiny perforations in the skin into which a dark dye is rubbed or injected to produce a visible pattern. Any body part that is relatively free of hair and remains fairly clean can be tattooed. Animals of all sizes can be tattooed, from small mammals to deer and bears ( Carnio and Killmar 1983 ; Downing and McGinnes 1969 ; Honma et al. 1986 ; Keith et al. 1968 ; Smythe 1978 ; Stirling 1989 ).
Conclusion
Research that involves trapping of mammals contributes to significant increases in our knowledge of evolution, ecology, animal behavior, physiology, parasitology, and genetics. Traps used in research should meet performance criteria that address state-of-the-art trapping technology and that optimize animal welfare conditions within the context of the research. Good research design should integrate ethics, performance criteria, techniques, and common sense, and IACUCs should address these topics when evaluating research protocols. Researchers must always work to improve research methods and to decrease the effects on research animals, if for no other reason that to minimize the chances that research methods affect the animals' behavior in ways that affect research results.
Mike Mitchell provided invaluable help with an early draft. Two anonymous reviewers and Michael Stoskopf provided additional comments.
References
Abbreviations used in this article: IACUC, institutional animal care and use committee; PIT, passive integrated transponder.



